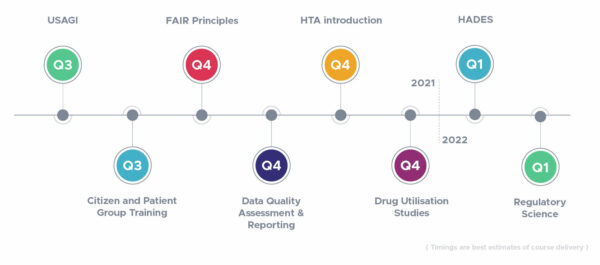
First rare disease registry that is FAIR from its conception is now online
All ERNs are tasked to set up patient registries that follow the FAIR Principles, as these ‘FAIR registries’ are essential for enabling efficient analysis of data across multiple sources. Existing methods to make clinical trial and registry data (more) machine-readable and FAIR are usually carried out after a research project is conducted and data are…